PRESS RELEASE
Diepenbeek, January 12th 2023
Beta-Cell NV reports the achievement of an important intermediate milestone in the generation of a xenogeneic off-the-shelf cell product for the treatment of diabetes.
Beta-Cell NV has established a freezing procedure by which its proprietary cell product (BetaprepTM) can be stored long term, while maintaining the ability to control diabetes.
In a comparative experiment in mice, Beta-Cell demonstrated equal diabetes reversal of cryopreserved cells compared to fresh cells. Furthermore, frozen cells even exceed fresh cells in their blood glucose controlling capacity in the initial study, indicating an enrichment of the more potent cells during the freezing process. These results do not only enable the generation of a superior graft for diabetes reversal, but are also an import step in the generation of a flexible off-the-shelf product for transplantation.
Cells can be produced at a centralized facility and preserved for future patient treatment in the clinic at remote locations worldwide. In addition, cryobanking allows sufficient time for elaborate quality control analysis and microbiological safety confirmation.
These new findings make the quality controlled BetaPrep product an attractive option to solve current issues of limited availability of human donor islets for diabetes patient transplantation. In summary, BetaPrep cryopreservation allows flexible manufacturing of a standardized and quality-controlled beta cell replacement therapy with high therapeutic potency for the treatment of diabetes.
Fabienne Soetaers, representative of Group Machiels as a main shareholder and board member of Beta-Cell NV comments: “In diabetes it is well established that a better regulation of blood glucose is key to prevent long-term diabetes complications that lead to a shorter life expectancy. Therefore, break-through treatments that ensure a more stable blood glucose level are eagerly awaited.”
Yanick Fanton, R&D Director of Beta-Cell: “With the significant progress in the field of genetically modified pigs for human therapeutic use, a revival of the xenotransplantation field is anticipated. Our demonstration that BetaPrep can be cryopreserved with full functionality opens the opportunity to develop an off-the-shelf cell product for diabetes. We are keen and committed to bring this xenotherapy solution to the patient as the unmet need of diabetes continues to grow in the upcoming decades.”
About Beta-Cell NV
Beta-Cell is Belgian biotech company that is developing a cell therapy for diabetes. The company has developed a quality controlled proprietary xenogeneic cell product and has an extensive track record in beta cell biology and therapeutic efficacy testing in diabetes models. The Beta-Cell porcine endocrine product shows diabetes reversal in animal models and key steps have been made to prepare for future clinical translation. The company is now focusing on finding partners with complementary expertise with respect to source pig genetic modification, cell encapsulation and/or other strategies for immune protection of transplanted grafts to enable clinical success. Beta-Cell has been funded by VLAIO, the European Union and by private investors, of which the Belgian-based Group Machiels serves as main shareholder.
For more information, please contact Beta-Cell NV, Yanick Fanton, R&D Director
yanick.fanton@beta-cell.com
www.beta-cell.com

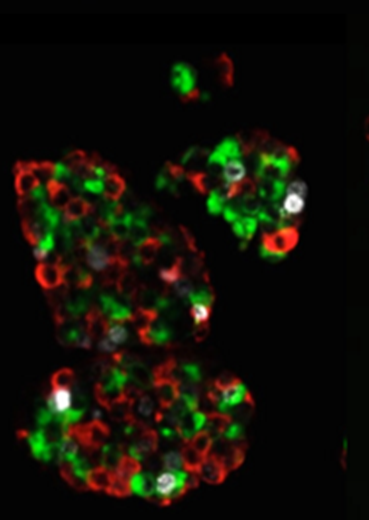
The BetaPrep islet cell product shown above is characterized by a high purity for all key endocrine cell populations to allow a tight control of the blood glucose level. Limited beta cell proliferation results in a therapeutic beta cell mass after transplantation. Insulin secreting beta cells are shown in red, glucagon secreting cells in green and proliferative cells in white.

